Hcl Acid Safety
Hydrochloric acidHCL Revision date. Irritating to eyes and respiratory system and skin.
Http Www Labchem Com Tools Msds Msds 75221 Pdf
If an acid is splashed onto bare skin rinse with water for at least 1520 minutes.

Hcl acid safety. HCl if not handled properly can be fatal and Personal Protective Equipment. Material Safety Data Sheet Product Name. Causes severe skin and eye burns and damage H314H318.
Browse Hydrochloric acid and related products at MilliporeSigma. Thermal decomposition generates. HYDROCHLORIC ACID HCl ALL GRADES _____ Safe Storage Conditions.
Keep locked up and out of reach of children. In addition it shows higher efficiency and uniformity of the dissolution of corrosion products and etching. SECTION 2 HAZARDS IDENTIFICATION Hazard class.
Hydrochloric acid HCl d 118 gcm 3 approximately 36 wt is a light yellow liquid. In the United States HCl is typically a tight-fill closed-loop loading operation and is loaded into trucks or rail cars via chemical hoses or by-pass arms. Its vapour has a detrimental effect on mucous membranes and is highly corrodible for the exposed instrumentation and equipment.
Hydrochloric acid is highly corrosive to most metals Exposure Routes inhalation ingestion solution skin andor eye contact. Hazardous Substances Data Bank HSDB Hydrochloric acid and hydrogen chloride react violently with many metals with the generation of highly flammable hydrogen gas which may explode. Keep away from heat sparks and open flames.
Hydrogen chloride solution Hydrochloric acid solution. Keep separated from incompatible. If an acid is splashed onto clothing consider removing the clothing immediately before the acid soaks through the clothing and reacts with the skin.
EEC CLASSIFICATION CXi Corrosive. Hydroxides amines alkalis copper brass zinc Note. Incompatible materials metals excess heat bases.
Bases amines metals permanganates eg potassium permanganate fluorine metal acetylides hexalithium disilicide. Reaction with oxidizers such as permanganates chlorates chlorites and. Bulk industrial-grade is therefore 30 to 35 optimized to balance transport efficiency and product loss through evaporation.
The aggressiveness of this acid is higher than that of the sulphuric acid. Widespread in many industrial and technical environments the hydrogen chloride detector whether fixed or portable can monitor HCl concentrations in different environmentsAfter having identified a dangerous concentration the HCl detector will alert its user in order to evacuate the dangerous zone. HAZARDOUS COMPONENTS Hydrochloric acid HCl is commercially available as a solution of up to 38 hydrogen chloride mm dissolved in water.
Skin and eye damage corrosion or irritation Category 1. CHEMFAB ALKALIS LIMITED Gnanananda place Kalapet Puducherry- 605014 India. Hazardous decomposition products Hydrogen chloride.
Substrates subjected to etching by means of hydrochloric acid. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. Ingestion of concentrated hydrochloric acid can cause severe injury to the mouth throat esophagus and stomach.
Manufacturer and Substance Identification. Keep container tightly closed. In the United States solutions of between 20 and 32 are sold as muriatic acid.
Store in a well-ventilated area. Hydrochloric acid is produced in solutions up to 38 HCl concentrated grade. Store in rubber-lined steel acid-resistant plastic or glass containers.
Store and handle in accordance with all current regulations and standards. STABILITY AND REACTIVITY Hydrochloric acid is stable under normal conditions and pressures. What is Hydrochloric Acid.
7647-01-0 UN. Hydrochloric Acid 5 vv Safety Data Sheet according to Federal Register Vol. Hydrochloric Acid also known as muriatic acid is an aqueous solution of hydrogen chloride.
Centers for Disease Control and Prevention CDC notes that hydrochloric acid can cause eye damage even blindness if splashed in the eyes. Higher concentrations up to just over 40 are chemically possible but the evaporation rate is then so high that storage and handling require extra precautions such as pressurization and cooling. Also known as hydrochloric acid as an aqueous solution hydrogen chloride or HCl is used.
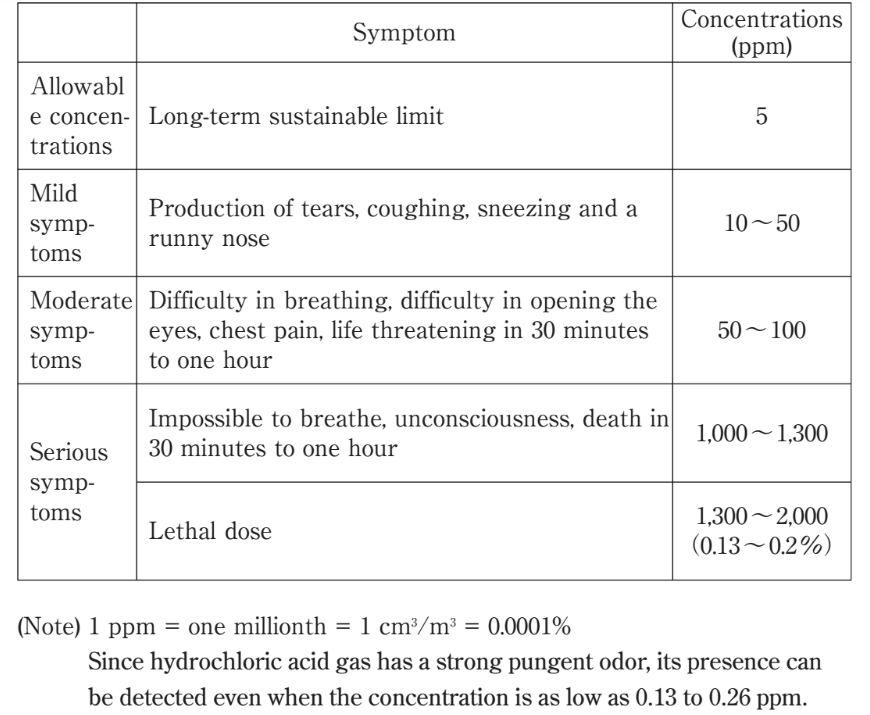
It is a colorless solution with a distinctively pungent smell. 1789 HAZCHEM2 R Formula. Inhalation of hydrochloric acid fumes produces nose throat and laryngeal burning and irritation pain and inflammation coughing sneezing choking sensation hoarseness laryngeal spasms.
Store in a cool dry area. If an acid splashes onto your skin and clothing immediately begin rinsing the affected areas with. 3645 Emergency Contact number.
Do not breathe mist vapors or spray P260. 58 Monday March 26 2012 Rules and Regulations 10242017 EN English US 58 105.
Hydrochloric Acid Acs Reagent 37 7647 01 0

Tiny Ghs Concentrated Hydrochloric Acid Label Sku Ghs 008 E

How Much Hydrochloric Acid Is Dangerous Quora

Small Ghs Concentrated Hydrochloric Acid Label Sku Ghs 008 C

Hcl Dvd Handling Hydrochloric Acid Safely Youtube

Warning Hydrochloric Acid Can Cause Severe Burns Label Sku Lb 2801
Msds Hcl Toxicity Dangerous Goods

Horizontal Nfpa Hydrochloric Acid Label Sku Lb 1592 066

Concentrated Hydrochloric Acid Ghs Sign Seton
Hydrochloric Acid Health Risks Vapor Mist And Fume Inhalation Sentry Air Systems Inc

Hydrochloric Acid Hazards Safety Tips Msdsonline
Http Science Cleapss Org Uk Resource Sss020 Hydrochloric Acid Pdf

Large Ghs Concentrated Hydrochloric Acid Label Sku Ghs 008 A

Ghs Chemical Labels Hydrochloric Acid Ghs Labels Seton

Hydrochloric Acid Stock Illustrations 212 Hydrochloric Acid Stock Illustrations Vectors Clipart Dreamstime

How To Safely Add Muriatic Acid To Your Pool Sensorex

8n Hydrochloric Acid Safety Data Sheet Fill Online Printable Fillable Blank Pdffiller
Msds Hcl Toxicity Dangerous Goods



Posting Komentar untuk "Hcl Acid Safety"